Calming the Cytokine Storm
CytoAgents’ drug, CTO1681, uses a novel approach to prevent and treat
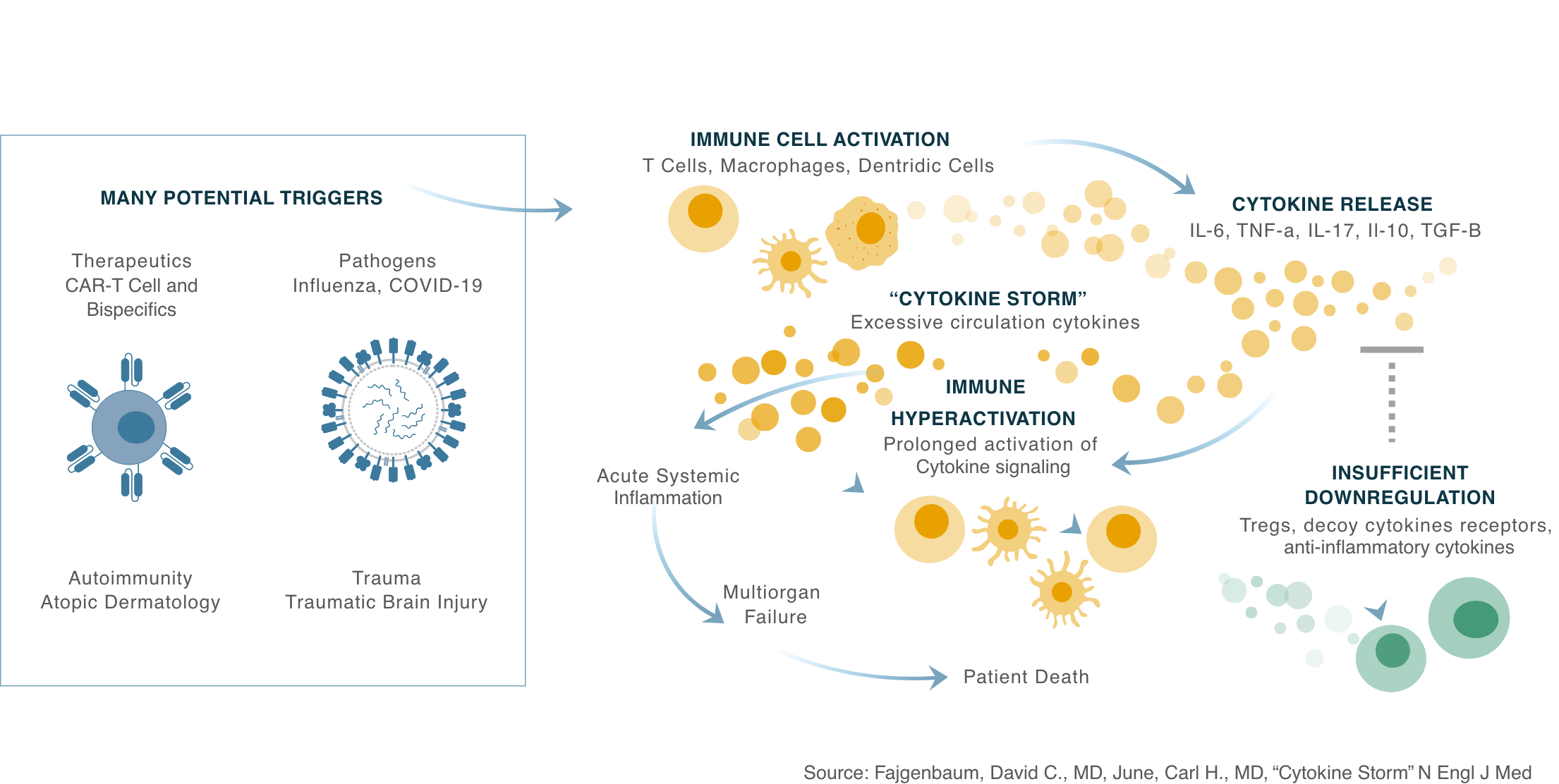
Cytokine Release Syndrome
How CTO1681 Works
The NF-kB pathway plays a critical role in the body’s natural regulation, activation, and differentiation of inflammatory T-cells. Excessive NF-kB actively results in pro-inflammatory cytokine amplification, as seen within Cytokine Release Syndrome, which contributes to the pathogenic processes of various inflammatory diseases.
CytoAgents’ drug candidate, CTO1681, prevents and treats Cytokine Release Syndrome by targeting the NF-kB signaling pathway via the activation of the EP4 receptor, which reduces NF-kB signaling, but does not shut it down completely. This, in turn, modulates cytokine production, resulting in reduced inflammation, while still allowing for a functioning immune system.
The Advantages of CTO1681
CTO1681 provides a number of advantages over other therapeutic options in treating CRS. By combining a broad mechanism of action to modulate cytokine production with a well-established safety record, it is positioned to become a new standard of care in the treatment of CRS.
- Phase 1 Human Clinical Trial Complete
- Well Established Safety Record
- Reduces 25+ Cytokines
- Prostaglandin Agonist, Anti-Inflammatory Mechanism of Action
- Steroid Sparing Solution
- Orally Available
Unlike other approaches, CTO1681 limits the potential damage caused by an overreaction of the immune system, but spares the patient from the potential of overly harsh immunosuppression associated with other potent therapies.
Our approach to treatment focuses on reducing the destructive impact of the CRS while leaving the necessary immune function intact.
